- Top
- Business
- Chemicals, food, biotechnology
- Natural ingredients
- Sesame peptide
- Sesame peptide clinical study results
Sesame peptide clinical study results
Dosage correlation exerting hypotension -1
- Purpose:
- Inspecting appropriate dosage by comparing the blood pressure measurement for diastolic pressure and systolic pressure with repeated oral administration of the test sample ‘Sesame peptide capsules KM-20’ known to have hypotensive effect, at dosage of 0mg, 250mg, 500mg, 1000mg per 4 capsules per day for 12 weeks to a group of outpatients who are normotensive to mild hypertensive.
- Conclusion:
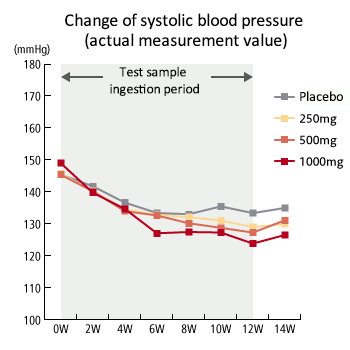
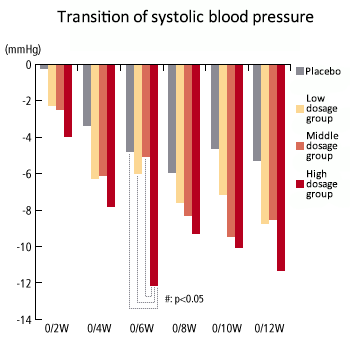
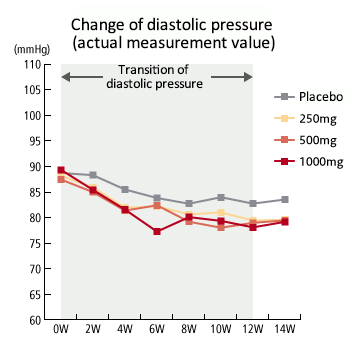
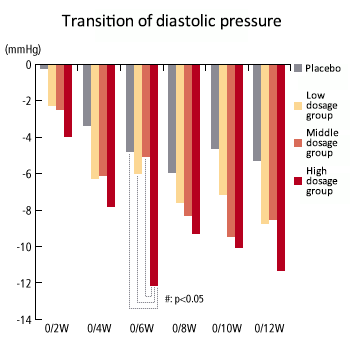
- The study demonstrated that among the high dosage taking group (1000mg), systolic blood pressure was decreased at the actual measurement value after the 6th week of administering test samples (sesame peptide KM-20). Also, systolic blood pressure decreased sequentially in the order of the 1000mg taking group, 500mg taking group, 250mg taking group and control group, indicating dose-dependency. These test results were taken into consideration and it was determined that medium (500mg/day) to high (100mg/day) dosage is suitable and a hypotensive effect can be expected.
| Testing facility: | Kunwakai Aiwa clinic medical institution |
|---|---|
| Testing period | December 21, 2002 to July 1, 2003 |
| Testing method: | Double blind test, parallel method between 4 groups The test period was divided into 1 week for pre-test observation, 12 weeks for the testing period, and 2 weeks for the post-test observation. |
| Number of candidates: | 60 (at the start of the test) 15 per group 58 (at pre-analytical stage) |
Dosage correlation exerting hypotension -2 (systolic blood pressure)
Dosage correlation exerting hypotension - 3 (diastolic pressure)
〒103-8410
11-2, Nihonbashi Honcho 4-chome, Chuo-ku, Tokyo, 103-8410
2nd Sales Division
Life Science Company
Food Material Team
TEL:03-3663-0274
FAX:03-3661-6459
- Business
-
- Synthetic resin
- Chemicals, food, biotechnology
- Electronics
- Packaging materials
- diX Coatings
 Synthetic resin
Synthetic resin Chemicals, food, biotechnology
Chemicals, food, biotechnology Electronics
Electronics Packaging materials
Packaging materials diX Coatings
diX Coatings