- Top
- Business
- Electronics
- Supercritical fluid technology
- Supercritical
Supercritical
A substance can have three states (solid, liquid and air) in general but a substance can be a supercritical fluid when it reaches above its critical point (Tc, Pc). This has the characteristics of both air (high diffusion) and liquid (melting ability). These unique characteristics can be used in the pharmaceutical and food industry, as a substitute of organic solvents which have a high burden on the environment. Also in the semiconductor and MEMS field, it is useful for cleaning ultrafine products and for being a drying solvent. This environmental-friendly new technology is attracting many interests.
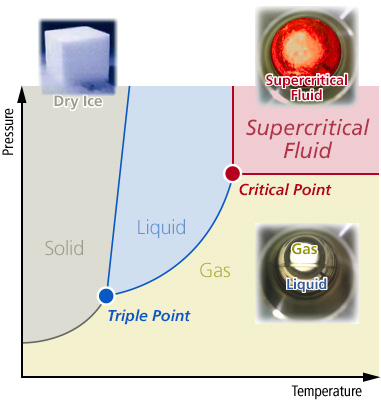
Characteristics of supercritical liquid
No surface tension
Compared with organic solvent, it has low surface tension and viscosity of carbon dioxide which enables it to spread and dry into ultrafine areas. In addition, there are no damages caused by drying because it can remove carbon dioxide without the presence of surfactant of air and liquid.
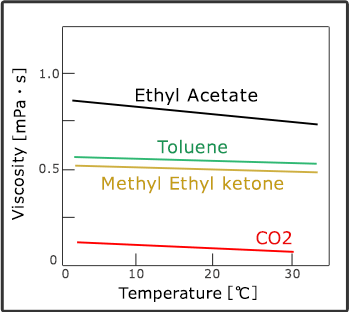
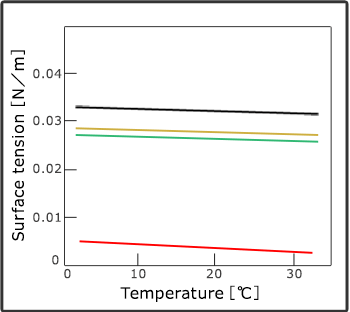
Melting and diffusion
Supercritical fluid has the characteristics of liquid (melting) and air (diffusion). Its physical property value reaches the middle point of liquid and air. Each molecule of carbon dioxide in particular has small interaction to one another, thus it can spread into superfine places and by increasing its density it can obtain more melting ability. For this reason, it had been applied as an extractant for a long time. In addition, it converts its melting ability significantly by controlling temperature and pressure which enables the extracted ingredients to be separated easily.
| Air | Supercritical Fluid | Liquid | |
|---|---|---|---|
| Density [kg/m3] | 0.6~1.0 | 200~900 | 1000 |
| Viscosity [Pa・s] | 10-5 | 10-5~10-4 | 10-4 |
| Diffusion coefficient [m2/s] | 10-5 | 10-7~10-8 | <10-9 |
Environment friendly
Carbon dioxide is safe as it is not poisonous, toxic nor flammable. The carbon dioxide used is the same as those that occur at thermal power stations, as they are gathered and refined. This technology can lead to immobilizing discharged carbon dioxide.
〒103-8410
11-2, Nihonbashi Honcho 4-chome, Chuo-ku, Tokyo, 103-8410
3rd Sales Division Semiconductor Material Department Development Section
TEL:03-3663-0276
FAX:03-3661-0037
- Business
-
- Synthetic resin
- Chemicals, food, biotechnology
- Electronics
- Packaging materials
- diX Coatings
 Synthetic resin
Synthetic resin Chemicals, food, biotechnology
Chemicals, food, biotechnology Electronics
Electronics Packaging materials
Packaging materials diX Coatings
diX Coatings